
Applied Clinical Trials
Pharmaceutical companies need to integrate pediatric assessments into the standard drug development process and build up pediatric competence.

Applied Clinical Trials
Pharmaceutical companies need to integrate pediatric assessments into the standard drug development process and build up pediatric competence.

Applied Clinical Trials
Weyers, Wolfgang (New York: Ardor Schribendi, Ltd., 2003) 755 pages.

Applied Clinical Trials
Orphan drug development may take a back seat to profit and loss concerns.

Applied Clinical Trials
Inadequate pre-approval testing and information disclosure may slow new drug development and change regulations.
Applied Clinical Trials
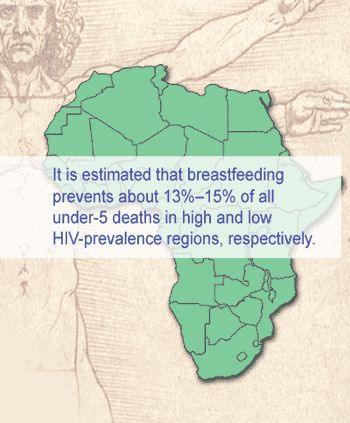
The data obtained by The Bellagio Child Survival Study Group has made the identification of priority research subjects more precise and relevant to developing countries.

Applied Clinical Trials
The Pediatric Rule made everyone begin to assess, think, and plan for pediatric needs.

Applied Clinical Trials
The traditional, "ideal" consent situation favors the immediate decision maker.

Applied Clinical Trials
Think of Teranode's modeling software program as the world's most sophisticated blackboard