
In this Q&A article, Professor Jenny Hewison of the University of Leeds shares her views on improving subject recruitment in the UK.

In this Q&A article, Professor Jenny Hewison of the University of Leeds shares her views on improving subject recruitment in the UK.

There's more than meets the eye to the EU's new public-centric initiative to simplify drug safety.
The 2006 Guidance reveals the advantages of using electronic modalities, such as IVR, when collecting patient-reported data.

Regulation will benefit both children & drug development, says EMEA, which has a lot on its plate this year.

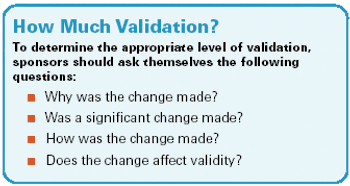
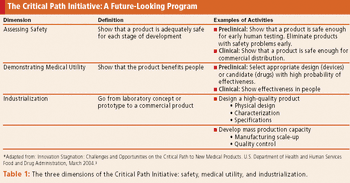
Drug safety concerns, unproductive methods, and high costs encourage innovative study designs and collaborative efforts.

The fate of a new bird flu vaccine that provides limited protection rests in the hands of FDA.
Interaction is high on FDA's list of ways to improve the development & review process for technologies.
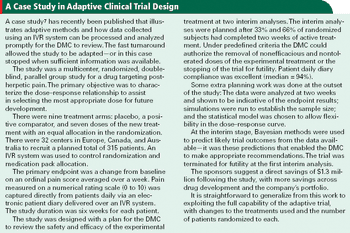
Interactive voice response systems are a good match for adaptive clinical trials and can help keep investigators in the dark.
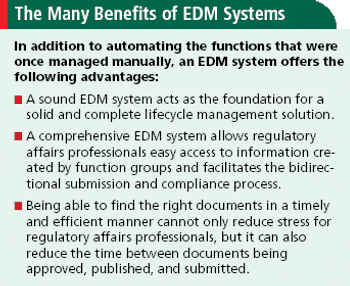
Why an electronic document management system is key to getting bought by major pharma.

Regulators and sponsors encourage alternative review models to fit a growing research enterprise.

With today's clinical investigators awash in regulatory burdens, it's high time sponsors provided a lifeboat.

UK report on last year's TGN 1412 trial provides industry with blueprint for improved early clinical studies.

A Q&A with members of CDISC and an executive in the software solution & services industry that explores the organization's efforts, capabilities & relationships.

Shift in Congressional control opens door to probes into research practices & expansion of PDUFA legislation.

AstraZeneca's interpretation of the EU Clinical Trial Directive: An industry perspective

Patient safety, orphan medicines featured in EU's six-year research support budget.

Safety issues come under close scrutiny at Amsterdam meeting.

A changing research enterprise requires clearer policies and more effective regulatory tactics.
Along with the titles and phone numbers of personnel, View from Brussels columnist Peter O'Donnell gives an update on the regulatory front in Europe.

This newly updated glossary includes even more terms relevant to clinical trials professionals.

This document from HHS and OHRP provides IRBs with instructions for reviewing trial information listed on Websites.

These translations help make sense of initials commonly used by clinical researchers around the world.

There's plenty to tackle this month, including a debate over efficacy, new ARDS guidelines, and evidence the EMEA is listening.

Under IOM's plan, a "lifecycle approach" to drug evaluation would change FDA's pre- and postapproval policies.

Michael Murphy, President and CEO of Gentris Corporation, answers questions about his company's recent expansion into Japan.