
The European Union is preparing a report on the lessons learned from its 2006 pediatric regulation.

The European Union is preparing a report on the lessons learned from its 2006 pediatric regulation.

An entire section of Horizon 2020 is devoted to health, demographic change, and well-being.


Clinical trials community reacts favorably to tentative set of new rules, yet questions remain.

Proposed rule changes on public information in Europe never make it to fruition.
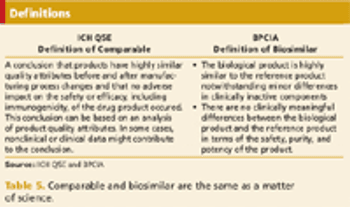
Biosimilars are a steadily growing new field of biopharmaceutical development and clinical research.

European Parliament voted against clearing the agency's accounts because of management concerns.

Multiple proposals for streamlining research emerge in legislation, expert reports.

Clinical trials should meet ethical and GCP standards no matter where in the world they are conducted.

Expanding access to nonprescription drugs and facilitating comparative effectiveness research.

Understanding the United States' regulatory pathways and clinical operations.

Demand for more information on study results and investigator payments create challenges.

The amendment addresses data concerns for both physicians and drug/device manufacturers.

The new Executive Director of the EMA has managed to stay calm under financial and political pressure.

Sponsors are responsible for monitoring studies, patient safety, and data integrity.

Congress, industry map out goals and concerns for revising FDA policies linked to user fee legislation.

European Forum for Good Clinical Practice meeting on informed consent draws a huge crowd.

Europe's struggling economies may have unexpected effects on the pharmaceutical sector.

FDA wants sponsors to build quality into protocols, adopt risk-based strategies to streamline trials.

A British Medical Journal survey suggests that misconduct in research is not going away.

Pressure to approve new user fees will affect policies for foreign studies and research methods.

While risk and uncertainty remain high, the pace of innovation will remain glacial.

The importance of safe use of imaging agents was highlighted at the Chicago Radiological Society of North America conference.

Patricia Brunko addresses the decline in European clinical trials, but still gives the industry hope.

The management board has a supervisory role with general responsibility for budgetary and planning matters, the appointment of the Executive Director, and the monitoring of the Agency's performance.