
While there's been hopeful news on treatments and vaccines, sponsors should plan to discuss necessary strategies and contingencies at the outset of new studies or re-opening of halted studies during the COVID-19 pandemic.

While there's been hopeful news on treatments and vaccines, sponsors should plan to discuss necessary strategies and contingencies at the outset of new studies or re-opening of halted studies during the COVID-19 pandemic.

IACT Health executive talks to Applied Clinical Trials about the importance of increased professional and technology investment at clinical trial sites.

With cloud-based software dominating data systems, on-premise installations still exist and command the pharmacovigilance software market.

Debunking the six most common myths regarding electronic informed consent.
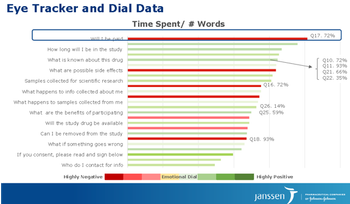
At ExL’s CROWN Congress, Cassandra Smith, Associate Director, Investigator & Patient Engagement at Janssen, discussed results from a study they conducted with patients on consent content modification.
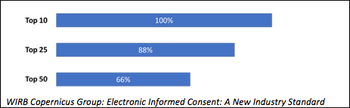
Debunking the six most common myths regarding electronic informed consent.

Peter O'Donnell discusses Estonia taking over the rotating presidency of the Council of the European Union.

As clinical trials have become more complex and costly, traditional paper-based data management systems have increasingly proved impractical and ineffective. Cloud-based technologies fit the bill, and industry professionals have begun to recognize this reality and reap the benefits at an ever-increasing rate.

CDISC executive talks about the creation of their cloud-based platform to free standards from PDF documents.

The challenges of data quality are a constant factor in clinical trials, especially that of traditional paper source documenting. Joyce Smith of The Medical Research Network speaks about the study’s site perspective on trial data quality.

The adoption of electronic health records by hospitals and providers has increased engagement in Health Information Technology (HealthIT) activities. Nora Belcher of the Texas eHealth Alliance speaks on how HealthIT will change the pharma landscape for drug development.

Clinical trials have changed significantly in the past decade with increasingly large and complex global studies. Multi-center, multinational trials are common, with complicated treatment protocols, large staffs, and huge data sets muddling the clinical trial processes. With years of development time and millions in R&D invested in a new treatment before it reaches the trial stage, there is incredible pressure on investigators to deliver quality data.

A recent eConsent survey reinforces the notion that this tech innovation is the next clinical trial solution the industry will adopt.

Adopting eConsent provides patients with adequate information on studies they may choose to participate in, and thus improving the rate of patient satisfaction and retention.

With the term "innovation" a buzzword du jour these days, the important quest for industry is to reimagine R&D using its new capabilities to apply real-world evidence, patient engagement, and collaborative technologies.

A technology-driven approach to oversight of vendors and sites can provide sponsors with timely and proactive solutions toward minimizing risks.
Roger DeRaad of Black Hills Cardiovascular Research and his staff discuss their experiences using IRT systems with 4G Clinical’s Kathleen Greenough. They offer insights into how sites, sponsors and technology vendors can cooperate to bring simplicity to clinical trials.

Electronic clinical outcome assessment (eCOA) devices have shown to be effective at avoiding missing data with a failure percentage at less than one percent. Paper back-up provisions produce a lesser value of clinical trials data captured and should be considered outdated.

This 3-part series presents results from a study of patient preferences regarding electronic Clinical Outcome Assessments in clinical trials. Part 3 covers how sponsors can humanize eCOA to help increase patient engagement during clinical trials.

This 3-part series presents results from a study of patient preferences regarding electronic Clinical Outcome Assessments in clinical trials. Part 2 covers how sponsors can improve upon their user interface of electronic diaries.

This 3-part series presents results from a study of patient preferences regarding electronic Clinical Outcome Assessments in clinical trials. Part 1 covers how sponsors can improve the study design and logistics of electronic diaries.

Technology platforms are today continually being adapted into the clinical trial life cycle with the promise of efficiency and reduced risk. CRF Health believes its combined eCOA/eConsent solution could improve the flow of documentation between investigators and sponsors.

The need to move beyond a point-solutions approach to one built around applications designed to manage the end-to-end clinical trial process is crucial.
Successful project management requires efficiency in both identifying and addressing bottlenecks before they impact study timelines. This article examines how to design data collection criteria for optimal performance.

Today’s convergence of health data and technology is fun to watch unfold, but a focused vision on system and process integration-and the framework for continuous enrichment-will be key to future lasting gains for clinical research.